 Jialing Xiang, professor of biology, and her collaborators have solved a longtime mystery: how protein kinase (Akt), a key player in cell signaling, “moves” from the cytosol (fluid inside of the cells) to the cell membrane.
Jialing Xiang, professor of biology, and her collaborators have solved a longtime mystery: how protein kinase (Akt), a key player in cell signaling, “moves” from the cytosol (fluid inside of the cells) to the cell membrane.
Using a state-of-the-art imaging tracking system, they discovered that a small protein called Ub14A can help to build “bridges,” called actin branches, that bring Akt directly to the cell membrane, where it gets activated. Their findings are published in an article in the July 20 issue of the Proceedings of the National Academy of Sciences (PNAS).
A major part of the work was done by Xiang’s Ph.D. student Yu Zhao as her doctoral thesis. Other collaborators included post-doctoral fellows and professors from the Department of Pharmacology, Yale University School of Medicine; and the Ben May Department for Cancer Research, University of Chicago.
Like a computer, most of our cells contain a complicated communication network that transmits cell signals (signaling transduction). It translates extracellular signaling into actions through a cascade process (signaling pathway). For example, the insulin signal tells cells to uptake more glucose so that we can keep a normal glucose level in blood.
Akt is one of the key components in the cell-signaling network and has been a major target for therapeutic intervention, especially for anticancer drug development, for decades. To perform as a signaling molecule, Akt must move from the cytosol to the cell membrane so it can be activated by its upstream components. After it is activated, it then quickly moves back to the cytosol so that it can direct its downstream “workers” in other cellular actions, such as cell growth, proliferation, energy metabolism, and even resistance to cancer.
“For years, scientists were puzzled as to how Akt ‘moves’ from the cytosol to the cell membrane,” said Xiang. “There is a ‘creek’ flowing underneath the cell membrane, and Akt is unable to ‘swim’ across by itself. Using both super resolution imaging and genetic approaches, we found that Ubl4A builds ‘bridges’ or actin branches that bring Akt directly to the cell membrane, where it gets activated.”
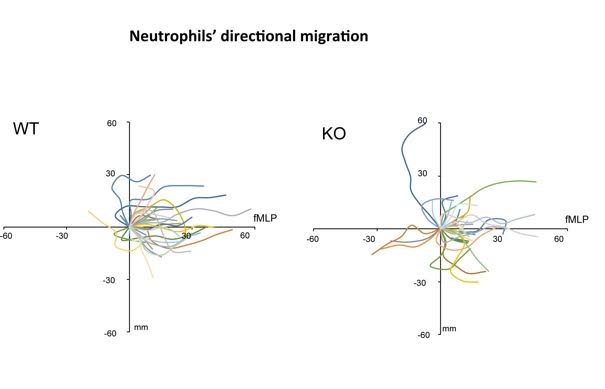
Mice deficient in Ubl4A show significant impairment in Akt signaling, resulting in, for example, high neonatal birth mortality and delay in growth. Actin branches also are important for many other cellular events, such as cell migration and wound healing. “For example, white blood cells normally migrate towards bacteria during infection, but white blood cells where Ub14A is absent get so confused in the direction that they cannot effectively reach the infection site,” Xiang said.
The study therefore both fills in a missing link in the Akt signaling pathway and uncovers a novel function of actin branching. It will have a broad impact in our understanding of the relationship between signaling and the actin network and provide a new spectrum of therapeutic targets for treatment of many related human diseases.
